45 ch2br2 hybridization
OneClass: What is the hybirdization of the C atom in CH2Br2? identify the hybridization of the c atom in ch2br2. rubyimpala346. Given the following reaction, how many g of CH2Br2 are formed if24.9 g Br2 react completely? ... CH2Br2 + 2 HBr A. 12.5 g CH2Br2 B. 13.5 g CH2Br2 C. 27.1 g CH2Br2 D. 8.13 X 1024 gCH2Br2. Fill in ALL of the required information. For each of the molecules below, please list the ... Is CH2Br2 Polar or Nonpolar? - Techiescientist The answer is yes. Here are some instructions to guide you: 1. Draw Lewis Structure. 2. With the application of VSEPR theory, find out the geometry of the molecule. 3. Look at the resultant dipole moment. 4. When the resultant dipole moment is 0, it is non-polar. If non-zero, it is polar. What is Polarity?
Hybridization - sp, sp2, sp3, sp3d, sp3d2 Hybridized Orbitals ... - BYJUS Hybridization in Chemistry is defined as the concept of mixing two atomic orbitals to give rise to a new type of hybridized orbitals. This intermixing usually results in the formation of hybrid orbitals having entirely different energies, shapes, etc. The atomic orbitals of the same energy level mainly take part in hybridization.
Ch2br2 hybridization
Identify the hybridization of the c atom in ch2br2. - Brainly.com The hybridization of the C atom in CH₂Br₂ is sp3 When bonding, the orbitals "s" and "p" from C atoms interact to form hybridized orbitals. If the C atom has 4 sigma bonds, as is the case in CH₂Br₂, there are 4 hybridized orbitals required, so 1 "s" orbital and 3 "p" orbitals hybridize to form a Unlock 15 answers now and every day CH2Br2 Molecular Geometry - Science Education and Tutorials The formula of CH2Br2 molecular hybridization is as follows: No. Hyb of CH2Br2= N.A (C-H and C-Br bonds) + L.P (C) No. Hy of CH2Br2= the number of hybridizations of CH2Br2 Number of C-H and C-Br bonds = N.A (C-Br and C-H bonds) Lone pair on the central carbon atom = L.P (C) Calculation for hybridization number for CH2Br2 molecule How to Determine the Hybridization of a Molecular Compound 1. Count the bonds and lone electron pairs connected to the atom. Iodine has 5 bonds and 1 lone electron pair. Each fluorine has 1 bond and 3 lone electron pairs. 2. Determine the hybridization. Since iodine has a total of 5 bonds and 1 lone pair, the hybridization is sp3d2. The exponents on the subshells should add up to the number of bonds ...
Ch2br2 hybridization. What is the electron geometry, molecular geometry, and hybridization of ... The electron geometry and molecular geometry are different. The arrangement of electron groups around the central atom whether bonding or non-bonding is known as the electron geometry. Molecular ... What is the hybridization about the central atom in C2Br2? - Answers The central atom is oxygen with sp3 hybridization. What type of hybridIzation is c2br2? There wont be a stable compound with the formula C2Br2. is then it will be sp hybridization of carbon. If the... Identify the orbitals that overlap to form the C-Br bonds in CH2Br2 a ... Overlap between various atomic orbitals produces hybrid orbitals. These hybrid orbitals have the same shape but dissimilar orientations. Answer and Explanation: 1 Become a Study.com member to... Dibromomethane | CH2Br2 | ChemSpider 20-52/53 Alfa Aesar A10456: 24-61 Alfa Aesar A10456: 6.1 Alfa Aesar A10456: DANGER: POISON, irritates skin, eyes, lungs Alfa Aesar A10456: H332-H412 Alfa Aesar A10456: P261-P273-P271-P304+P340-P312-P501a Alfa Aesar A10456: Safety glasses, adequate ventilation.
Dibromomethane | CH2Br2 - PubChem Dibromomethane | CH2Br2 | CID 3024 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. National Institutes of Health. National Library of Medicine. National Center for Biotechnology Information. PubChem ... C2H2Br2 Lewis Structure: How to Draw the Lewis Structure for ... - YouTube A step-by-step explanation of how to draw the C2H2Br2 Lewis Dot Structure (1,2-Dibromoethylene).For the C2H2Br2 structure use the periodic table to find the ... CH2Cl2 lewis structure, molecular geometry, polarity | Dichloromethane Hybridization of Dichloromethane When two or molecules participate in the bond formation, their orbitals overlap due to the sharing of electrons. These overlapped orbitals are called hybrid orbitals. The bonds formed in Dichloromethane are covalent bonds. Central Carbon is hybridized as the molecule forms all the four bonds in the compound. Schupf Computational Chemistry Lab - Colby College The Lewis structure that is closest to your structure is determined. The hybridization of the atoms in this idealized Lewis structure is given in the table below. Hybridization in the Best Lewis Structure. 1. A bonding orbital for C1-H2 with 1.9965 electrons __has 63.66% C 1 character in a sp2.32 hybrid __has 36.34% H 2 character in a s orbital. 2.
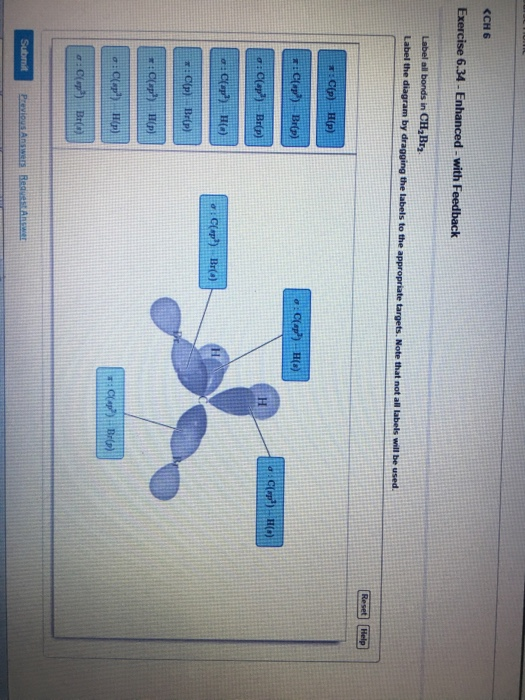
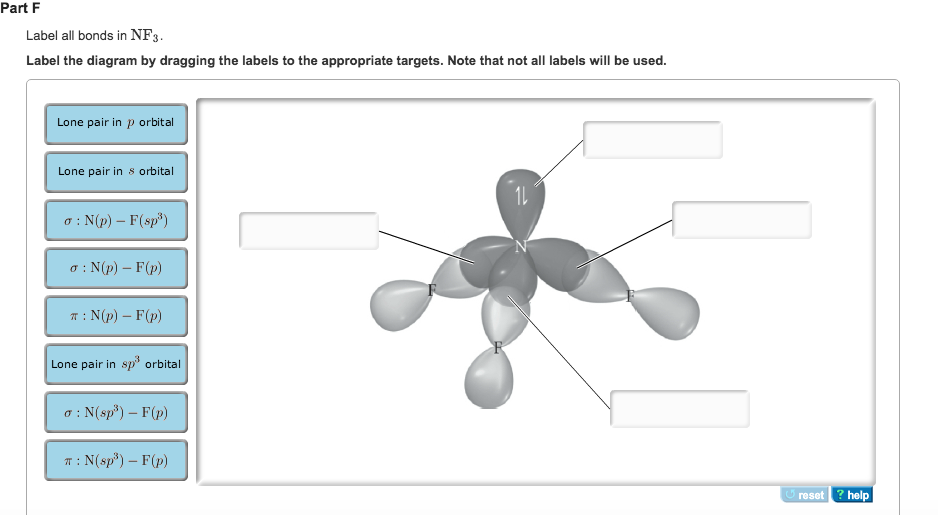
Hybridization of Carbon - Molecular Geometry and Bond Angles A carbon atom is sp2 hybridized when bonding takes place between 1 s-orbital with two p orbitals. There is a formation of two single bonds and one double bond between three atoms. The hybrid orbitals are placed in a triangular arrangement with 120° angles between bonds. Example: Hybridization of graphite 3. sp3 Hybridization Finding the hybridization of atoms in organic molecules (worked ... SAP‑4.C.4 (EK) Transcript. We can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. In this video, we use both of these methods to determine the hybridizations of atoms in various organic molecules. Created by Jay. SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. CH2Br2 b. SO2 c. NF3 d. BF3. Answer (a) See solution (b) See solution (c) See solution (d) See solution. View Answer. Related Courses. Chemistry 101. Chemistry ... CH2Br2 Lewis Structure, Geometry, Hybridization, and Polarity In CH2Br2, C is the central atom. We are focusing on the hybridization of the central atom only. In the ground state, C has 2 unpaired electrons. It can only form two bonds. Promotion of electrons takes place, and all 4 valence electrons become unpaired. These 4 electrons are present in different orbitals.
Solved 1.Identify the hybridization of the C atom in | Chegg.com See the answer 1.Identify the hybridization of the C atom in CH2Br2. 2. Identify the hybridization of the S atom in SO2. 3. Identify the hybridization of the N atom in NF3. 4. Identify the hybridization of the B atom in BF3. Expert Answer 100% (102 ratings) Previous question Next question
What hybridization is expected on the central atom of each of the ... H= Number of orbitals involved in hybridization. V=Valence electrons of central atom. M- Number of monovalent atoms linked to central atom. C= Charge of cation. A= Charge of anion. Consider the hybridization of the options:-A) B e H 2 -H = 2 1 [2 + 2] = 2. ⇒ s p hybridized state. B) C H 2 B r 2 H = 2 1 [4 + 4] = 4. ⇒ s p 3 hybridized state ...
What type of hybridIzation is c2br2? - Answers Best Answer. Copy. There wont be a stable compound with the formula C2Br2. If there is then it will be sp hybridization of carbon. If the question is for CH2Br2, then carbon will be sp3 hybridized ...
CHEM: Chapter 10 Flashcards | Quizlet The sp3 and sp3d2 hybridization schemes have no unhybridized p-orbitals left to form π-bonds. Valence Bond Theory 62. Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CH2Br2 b. SO2 c. NF3 d. BF3
dentify the hybridization of the C atom in CH2Br2. Identify the ... The central atom is generally considered to be the electrostatic central atom. Calculate hybridization as follows: 1. If the value of X is 2 then it means that two hybrid orbitals are to be formed and the hybridization of the sp. 2. If X is a value of 3 then it means that three hybrid orbitals are to be formed and that they are hybridized . 3.
CH2Br2 Lewis Structure: How to Draw the Lewis Structure for ... - YouTube A step-by-step explanation of how to draw the CH2Br2 Lewis Dot Structure (Dibromomethane).For the CH2Br2 structure use the periodic table to find the total n...
How to Determine the Hybridization of a Molecular Compound 1. Count the bonds and lone electron pairs connected to the atom. Iodine has 5 bonds and 1 lone electron pair. Each fluorine has 1 bond and 3 lone electron pairs. 2. Determine the hybridization. Since iodine has a total of 5 bonds and 1 lone pair, the hybridization is sp3d2. The exponents on the subshells should add up to the number of bonds ...
CH2Br2 Molecular Geometry - Science Education and Tutorials The formula of CH2Br2 molecular hybridization is as follows: No. Hyb of CH2Br2= N.A (C-H and C-Br bonds) + L.P (C) No. Hy of CH2Br2= the number of hybridizations of CH2Br2 Number of C-H and C-Br bonds = N.A (C-Br and C-H bonds) Lone pair on the central carbon atom = L.P (C) Calculation for hybridization number for CH2Br2 molecule




Post a Comment for "45 ch2br2 hybridization"