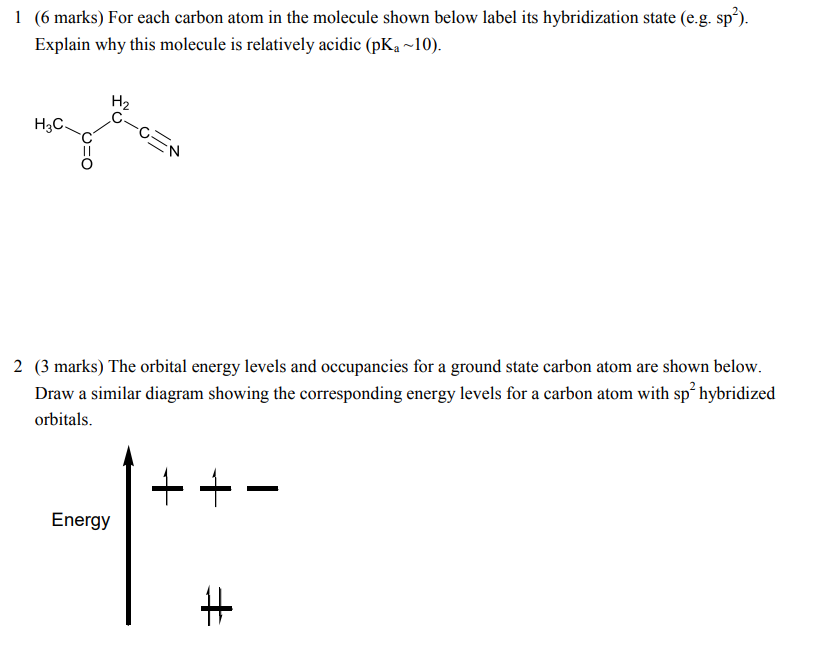
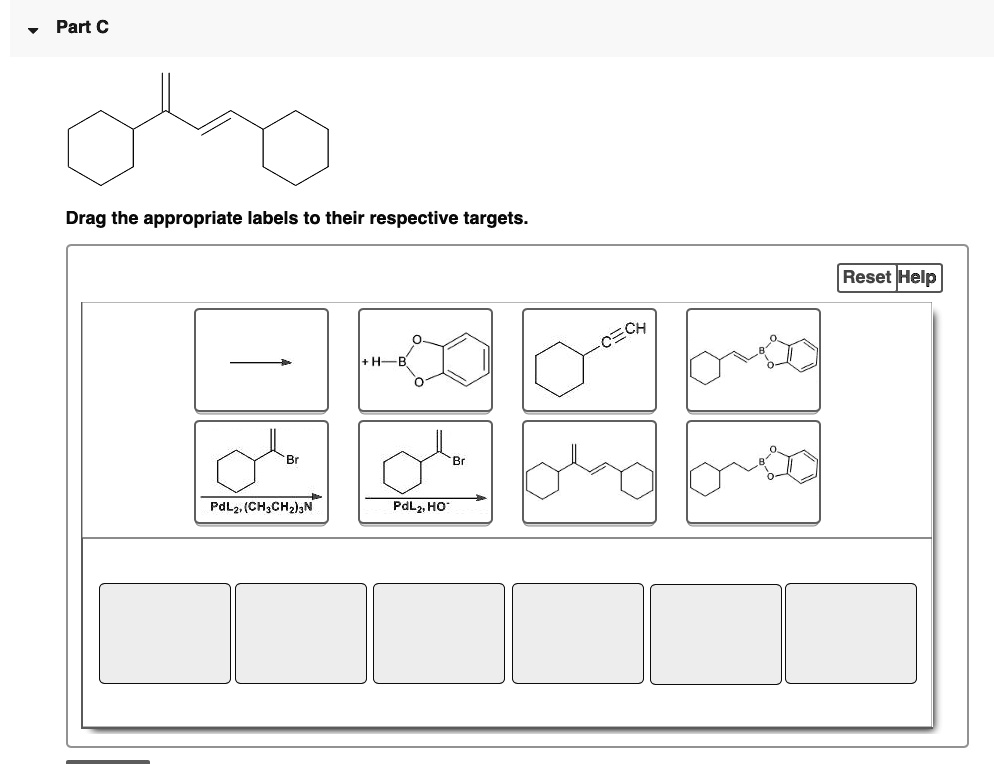
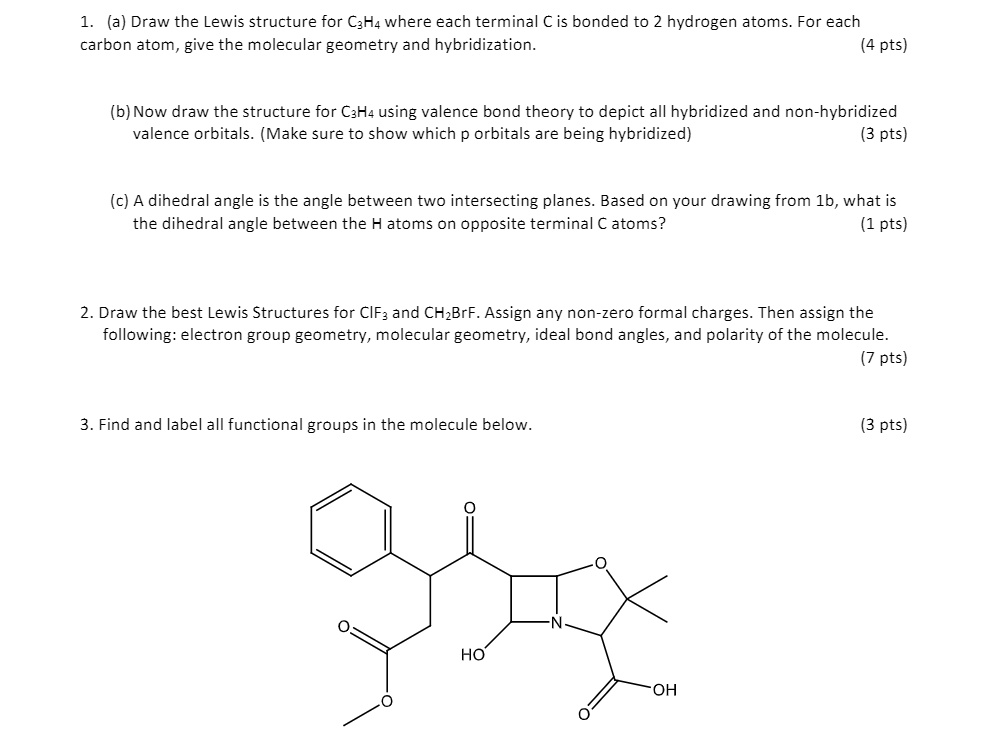
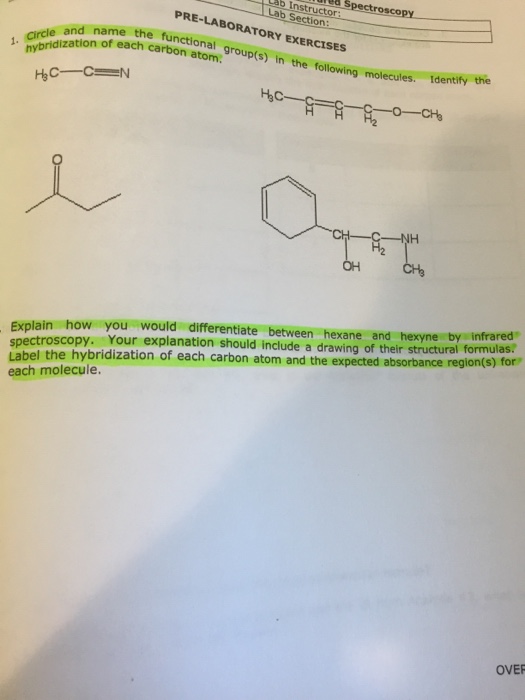
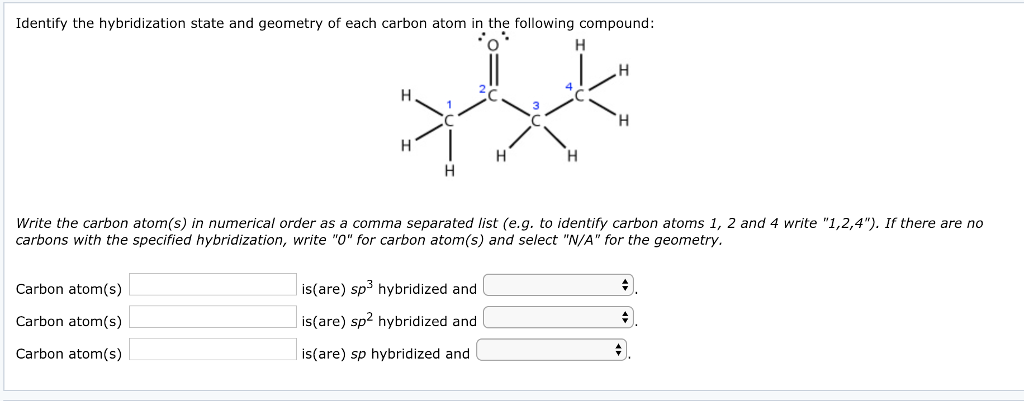
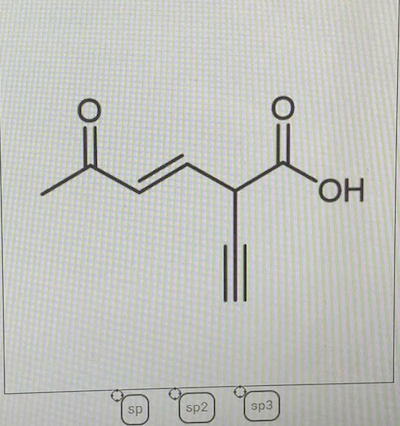
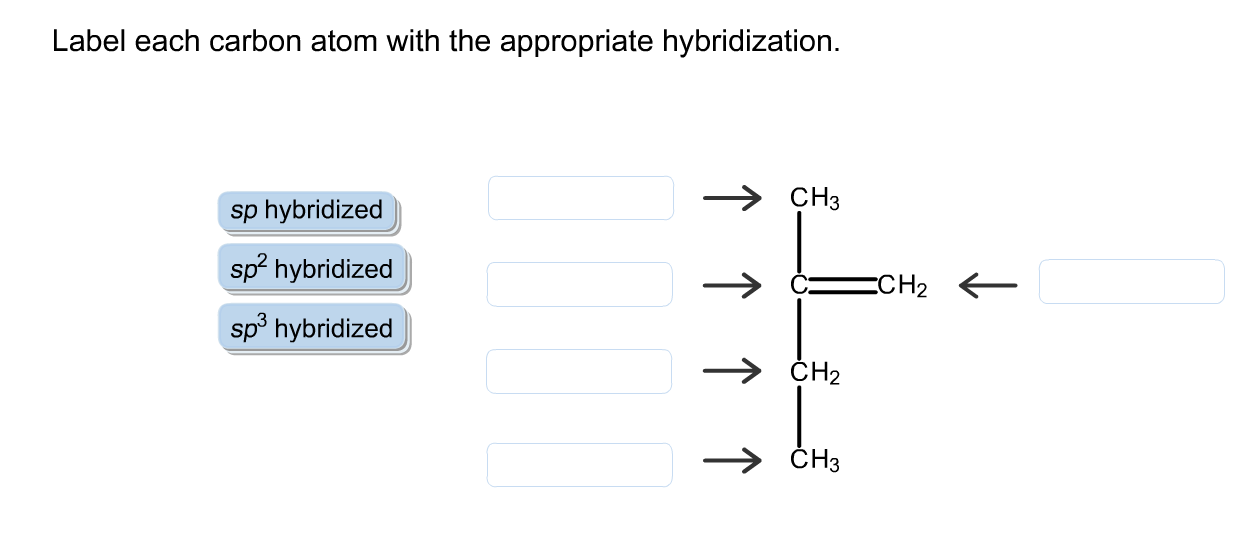
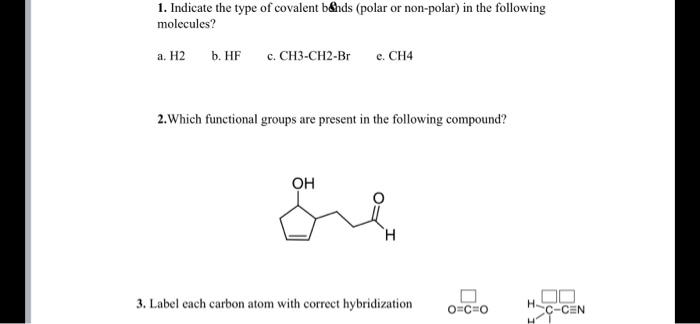
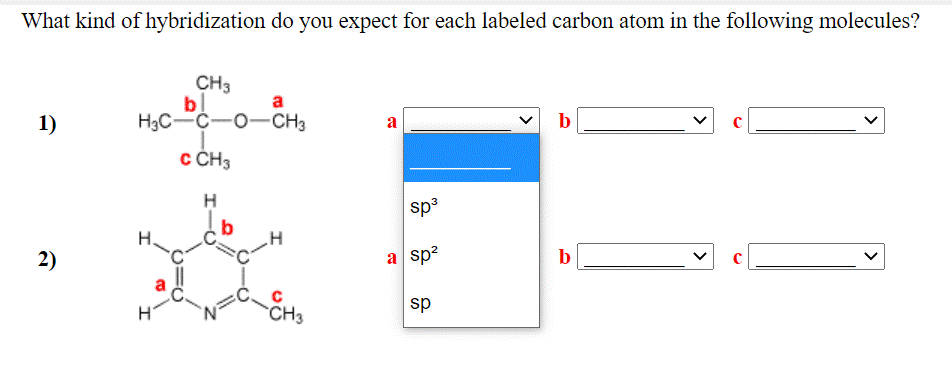
44 label each carbon atom with the appropriate hybridization
Ochem Exam 1 abridged Flashcards | Quizlet 1 carbon CH₄ ... Ethane ... how many carbons ... write formula 2 carbons CH₃CH₃ ... Propane ... how many carbons ... write formula 3 carbons CH₃CH₂CH₃ ... Butane ... how many carbons ... write formula 4 carbons THIS SET IS OFTEN IN FOLDERS WITH... OChem exam 2 abridged (starred only) 109 terms luzangelica96 OChem exam 2 abridged (updated study all) Label each carbon atom with the appropriate hybridization. CH ... In the given question we have to label each carbon atom appropriate hybridization. This is asked in the given right the structure first and then apply the label to each carbon atom. The keynote here is we have to identify the appropriate hybridization of carbon atoms. Given industry. Let's make the structure has given in the question CH three.
Solved Label each carbon atom with the appropriate | Chegg.com Question: Label each carbon atom with the appropriate hybridization. Show transcribed image text Expert Answer 98% (57 ratings) The top one is SP3 hybridized, having three sp3 bonds w hydrogen and … View the full answer Transcribed image text: Label each carbon atom with the appropriate hybridization. Previous question Next question
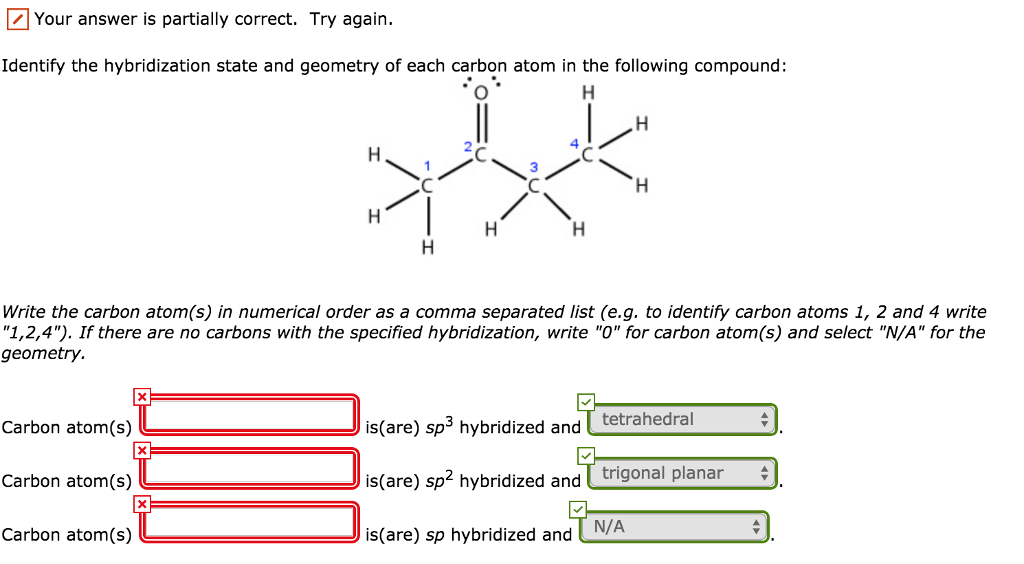
Label each carbon atom with the appropriate hybridization
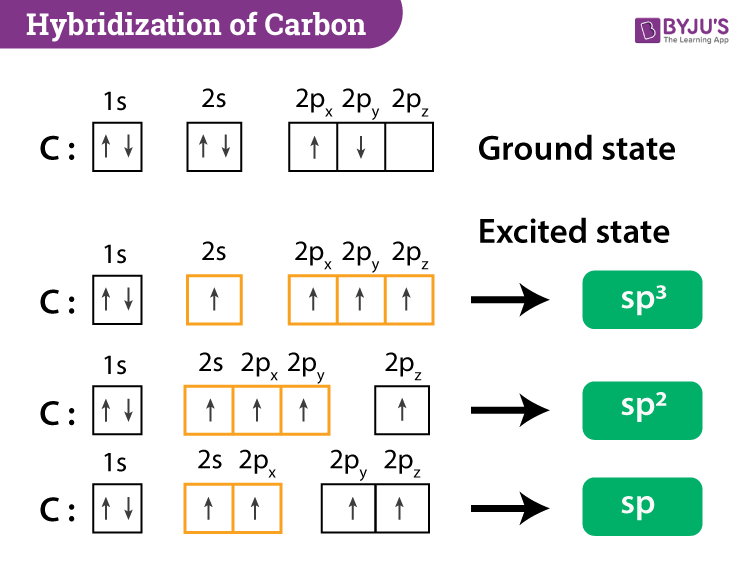
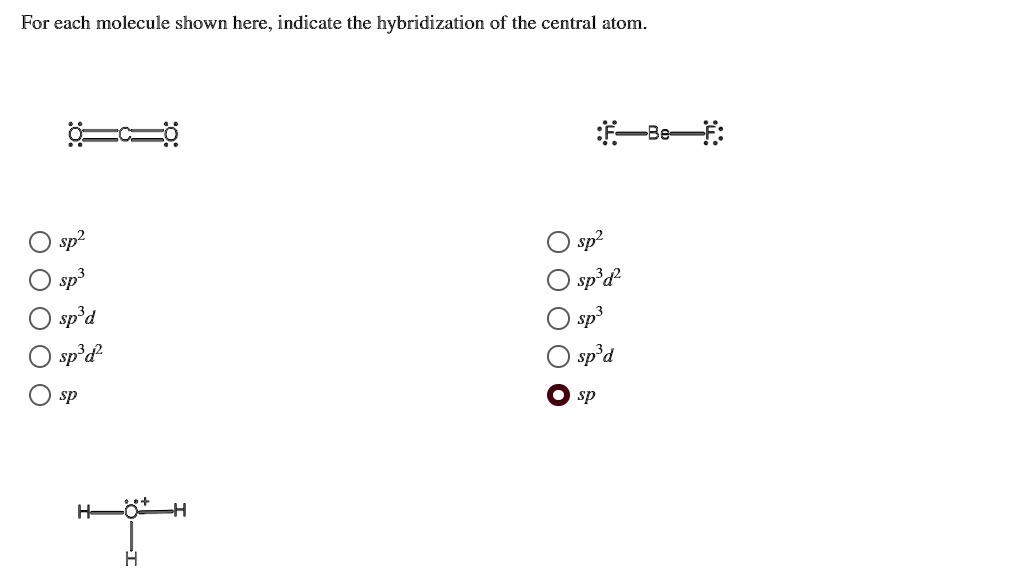
(Get Answer) - Label each carbon atom with the appropriate ... Label each carbon atom with the appropriate hybridization. sp,sp2 or sp3 Label each carbon atom with the appropriate hybridization. sp,sp2 or sp3 Nov 18 2022 08:12 AM Hybridization of Carbon - Molecular Geometry and Bond Angles - BYJUS Carbon can have an sp hybridization when it is bound to two other atoms with the help of two double bonds or one single and one triple bond. When the hybridization occurs the molecules have a linear arrangement of the atoms with a bond angle of 180°. Example: Hybridization of CO 2. 2. sp2 Hybridization What are hybridisation states of each carbon atom in the following ... The hybridisation states of each carbon atom in the following compounds are given below. ... sp2 Hybridization. 10 mins. sp3, sp3d and sp3d2 Hybridization. 22 mins. sp3d3 Hybridization. 11 mins. Shortcuts & Tips . Problem solving tips > Mindmap > Important Diagrams > Common Misconceptions >
Label each carbon atom with the appropriate hybridization. Quickly Determine The sp3, sp2 and sp Hybridization - Chemistry Steps Other methods to determine the hybridization. In addition to this method, it is also very useful to remember some traits related to the structure and hybridization. In general, an atom with all single bonds is an sp 3 hybridized. The best example is the alkanes. All the carbon atoms in an alkane are sp 3 hybridized with tetrahedral geometry. (b) What is the hybridization of the carbon atoms in each molecul... (b) What is the hybridization of the carbon atoms in each molecule? ... 12. Molecular Shapes & Valence Bond Theory · Hybridization. Problem ... Solved Label each carbon atom with the appropriate | Chegg.com Question: Label each carbon atom with the appropriate hybridization. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Show transcribed image text Expert Answer 100% (40 ratings) sp3 … View the full answer OChem Spring 2017 Exam 1 Flashcards | Quizlet Label each carbon atom with the appropriate hybridization (cover bottom) ... Sapling Hw Ch 1.18. a) 120° b) 109.5° Label each carbon atom with its optimum C-C-C bond angle (cover bottom) Sapling Hw Ch 1.19. Rank the following compounds according to increasing positive character of the carbon atom CH₃F
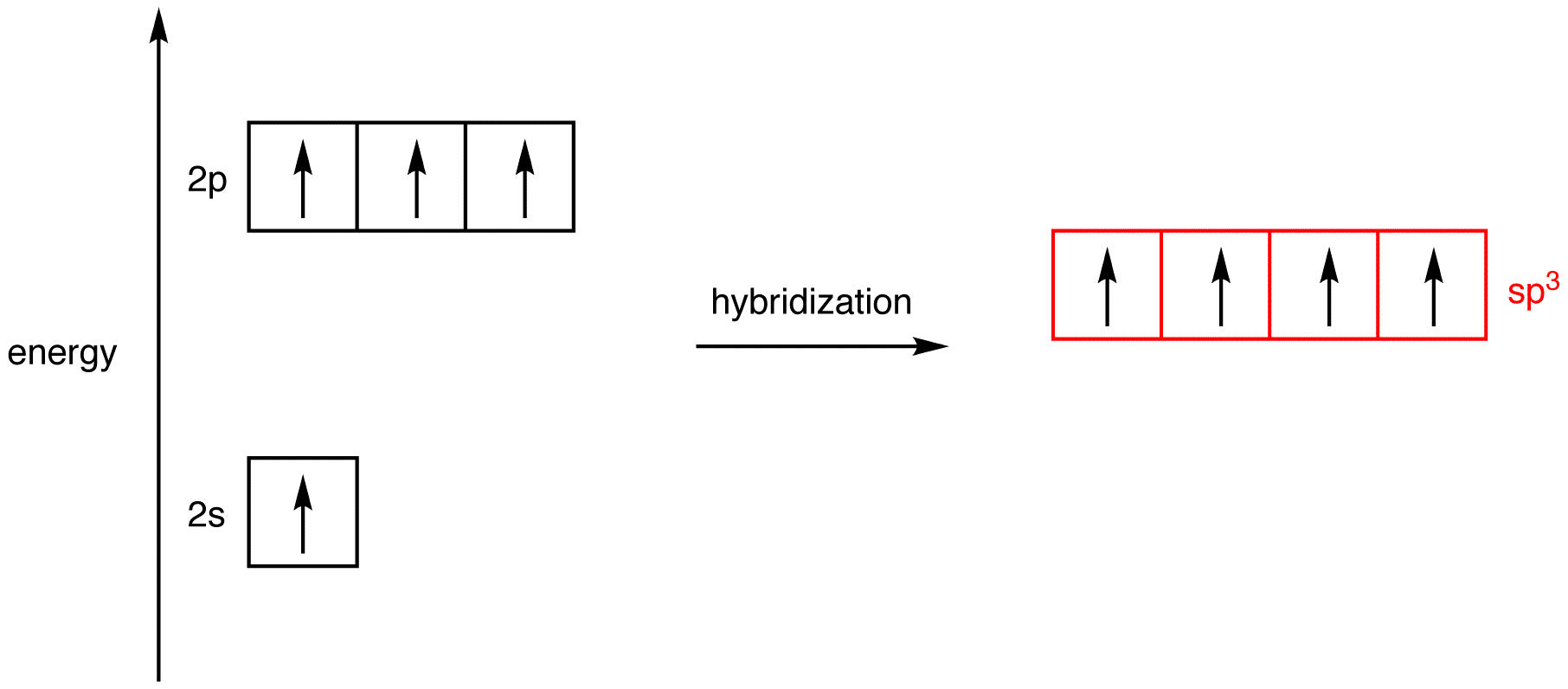
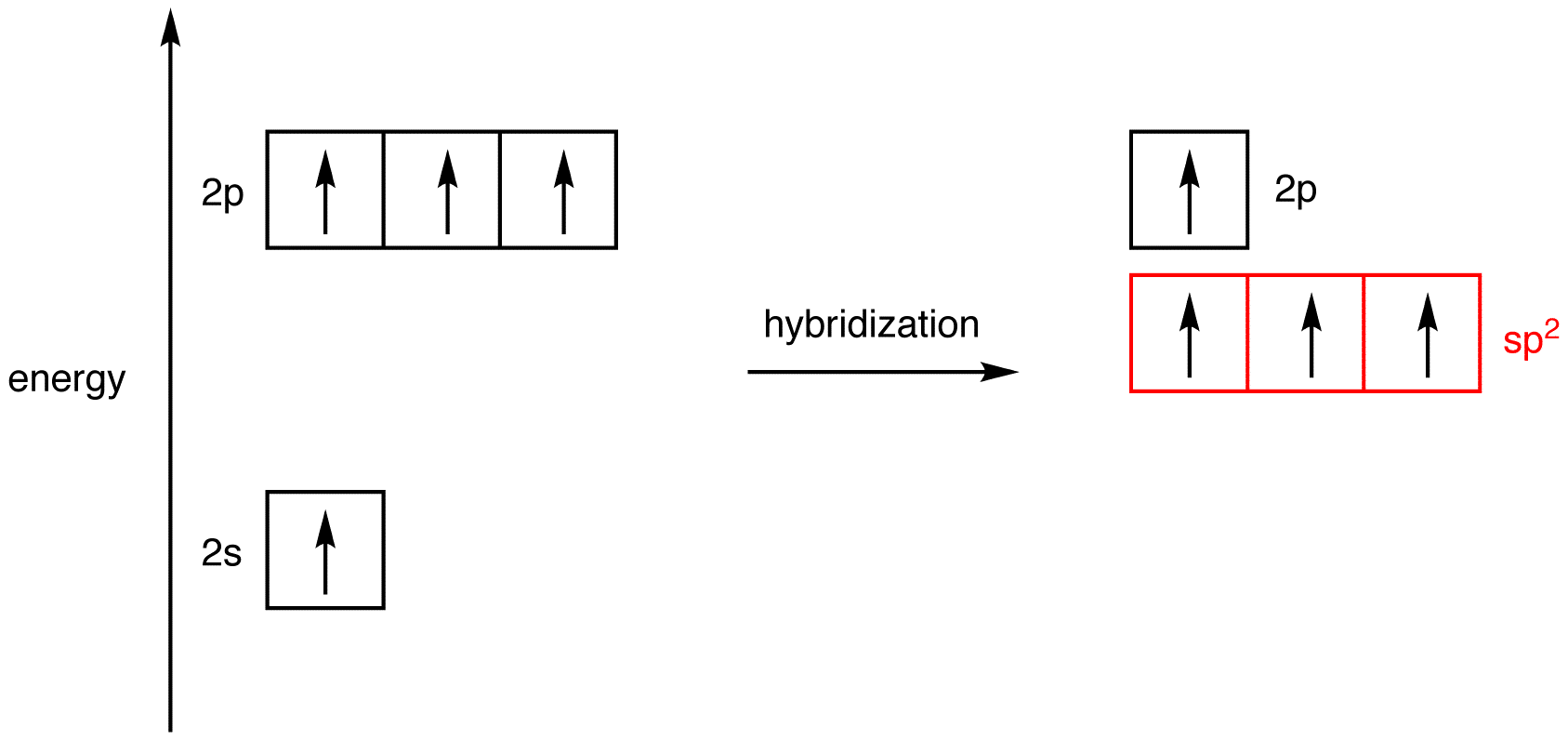
Label each carbon atom with the appropriate hybridization.sp,sp2 or ... Get the detailed answer: Label each carbon atom with the appropriate hybridization.sp,sp2 or sp3 Label each carbon atom with the appropriate hybridization. sp³ hybridization | Hybrid orbitals | Chemical bonds (video) | Khan Academy AboutTranscript. In sp³ hybridization, one s orbital and three p orbitals hybridize to form four sp³ orbitals, each consisting of 25% s character and 75% p character. This type of hybridization is required whenever an atom is surrounded by four groups of electrons. Created by Jay. Sort by: Finding the hybridization of atoms in organic molecules (worked ... We can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. In this video, we use both of these methods to determine the hybridizations of atoms in various organic molecules. Created by Jay. Sort by: Top Voted Questions Tips & Thanks What is the hybridization of each carbon atom in acetonitrile? This will account for. 4 × 2 e− +1 × 6 e− = 14 e−. The remaining 2 valence electrons will be added on the nitrogen atom as a lone pair. In order to find the hybridization of the two carbon atoms, you must count the regions of electron density that surround the atoms. A region of electron density is simply. a single, double, or triple bond.
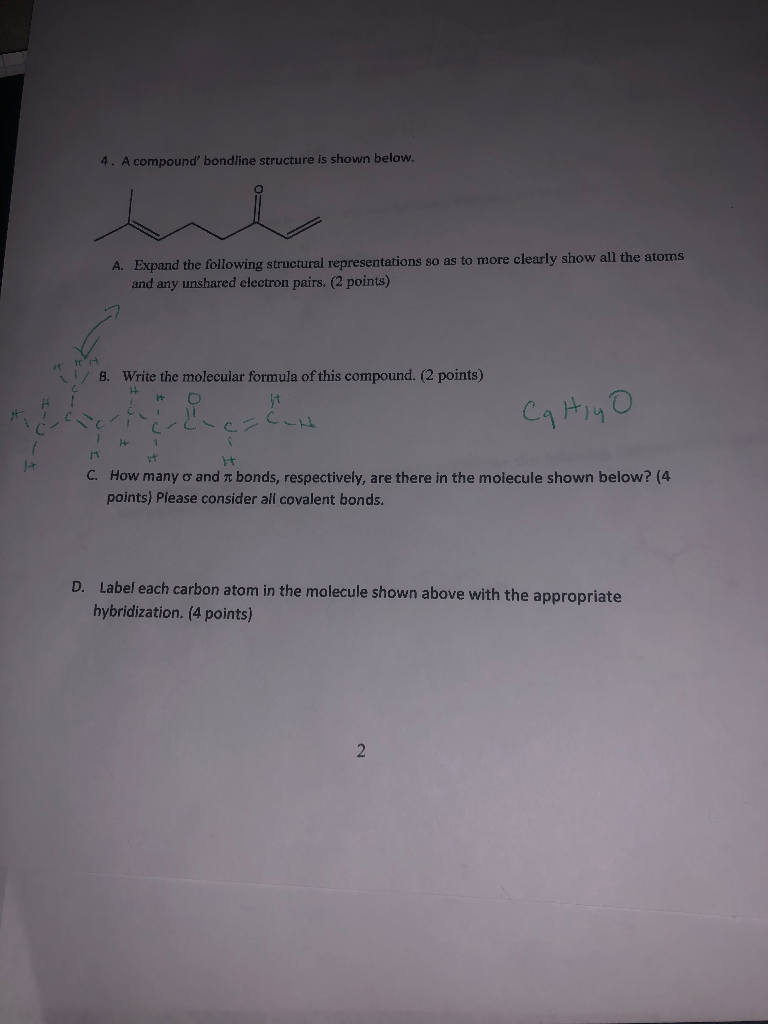
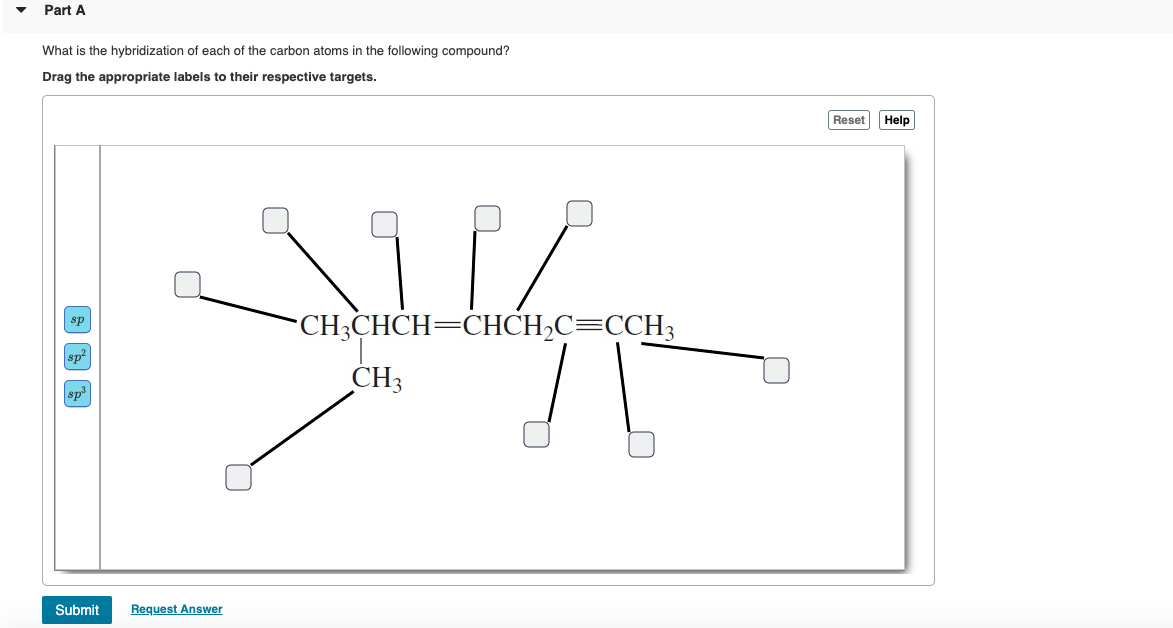
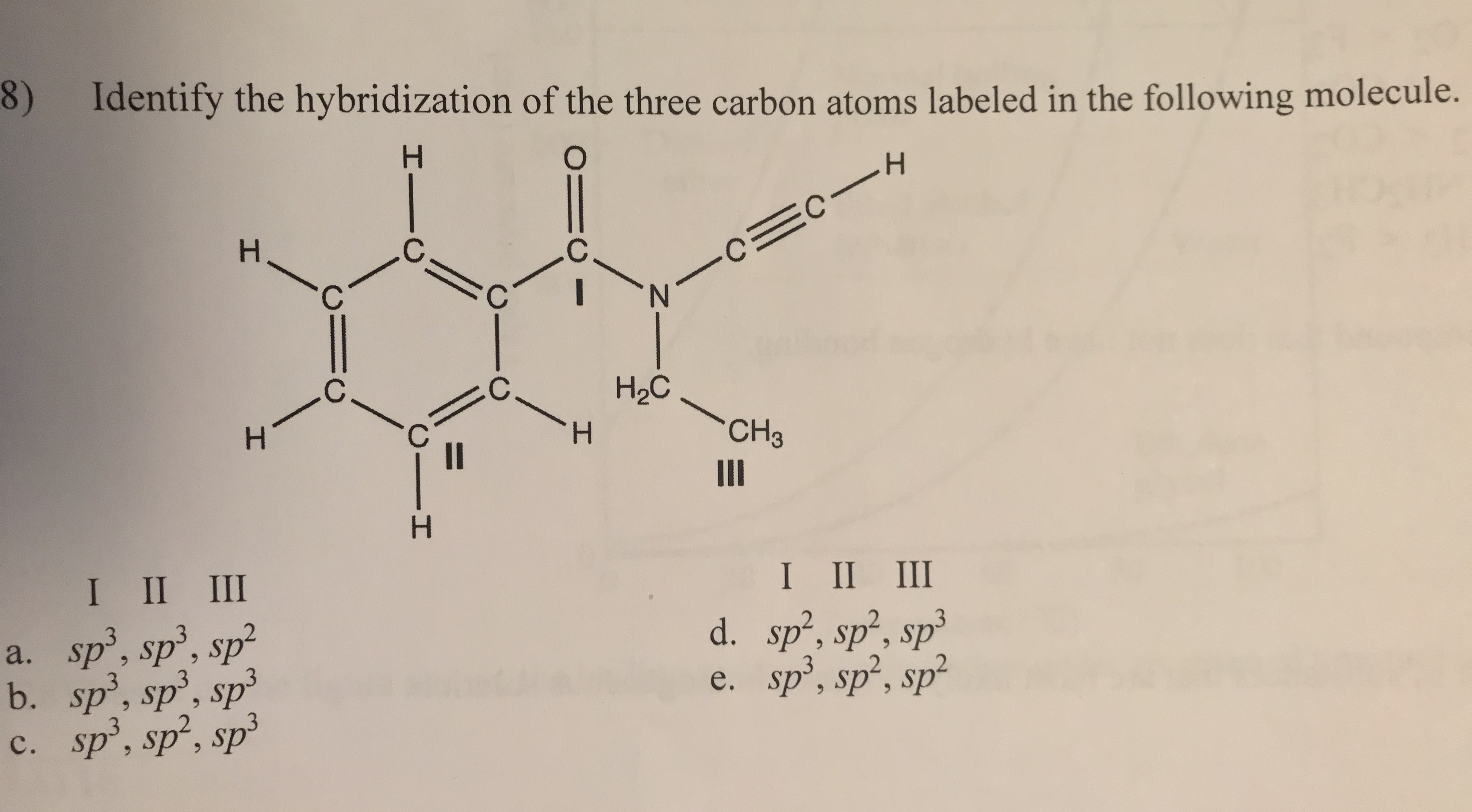
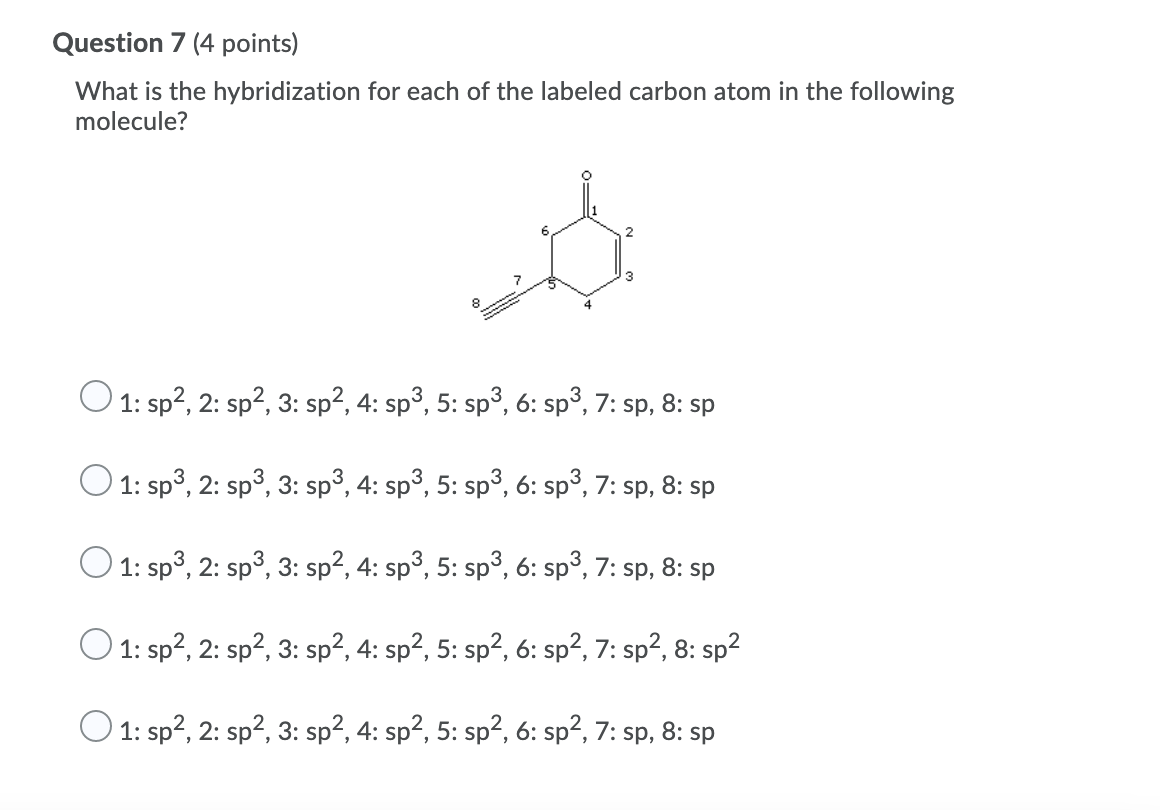
Chapter 9 Homework Flashcards | Quizlet There are 6 C atoms in the molecule. Starting on the left, the hybridizations are: sp2, sp2, sp3, sp, sp, sp3. All single bonds are bonds. Double and triple bonds each contain 1 bond. This molecule has 8 C-H bonds and 5 C-C bonds, for a total of 13 bonds. Double bonds have 1 bond and triple bonds have 2 bonds. This molecule has a total of 3 bonds. What is the hybridization of the carbon atom labeled 4? - Toppr For this molecule, carbon sp2 hybridises, because one π bond is required for the double bond between the carbons and only three σ bonds are formed per carbon ... Write a hybridization and bonding scheme for each molecule or io... Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. d. I3-. Show Answer. Relevant ... Hybrid Orbitals - Chemistry LibreTexts To form four bonds the configuration of carbon must have four unpaired electrons. One way CH 4 can be explained is, the 2s and the 3 2p orbitals combine to make four, equal energy sp 3 hybrid orbitals. That would give us the following configuration: Now that carbon has four unpaired electrons it can have four equal energy bonds.
5.3: Hybridization of Atomic Orbitals - Chemistry LibreTexts Three atomic orbitals on each carbon - the 2 s, 2 px and 2 py orbitals - combine to form three sp 2 hybrids, leaving the 2 pz orbital unhybridized. The three sp 2 hybrids are arranged with trigonal planar geometry, pointing to the three corners of an equilateral triangle, with angles of 120°between them.
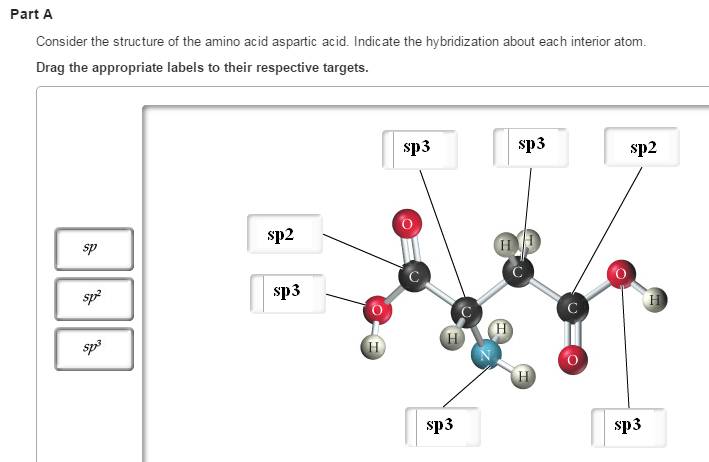
How To Determine Hybridization: A Shortcut - Master Organic Chemistry Here's a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in Org 1. For a given atom: Count the number of atoms connected to it (atoms - not bonds!) Count the number of lone pairs attached to it. Add these two numbers together. If it's 4, your atom is sp3.
label each carbon atom with the appropriate hybridization Get the detailed answer: label each carbon atom with the appropriate hybridization OneClass: label each carbon atom with the appropriate hybridization 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION →

What are hybridisation states of each carbon atom in the following compounds? CH2 = C = O, CH3CH = CH2, (CH3)2CO, CH2 = CHCN, C6H6
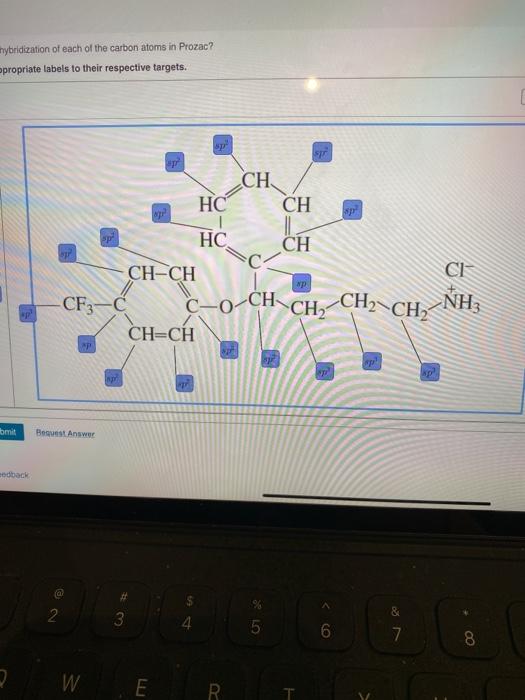
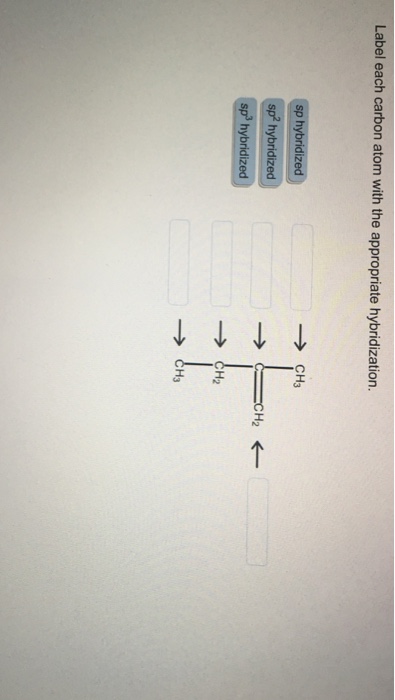
Answered: of 20 > Label each carbon atom with the… | bartleby Solution for of 20 > Label each carbon atom with the appropriate hybridization. sp' hybridized CH3 Answer Bank sp hybridized sp hybridized ECH, ...
label each carbon atom with the appropriate hybridization - YouTube label each carbon atom with the appropriate hybridization - YouTube 0:00 / 1:54 label each carbon atom with the appropriate hybridization 457 views Jun 11, 2020 5 Dislike Share Save OneClass...
Label each carbon atom with the appropriate hybridization. sp ... To apply the label to each carbon atom, the structure needs to be asked first. We have to identify the best way to mix carbon atoms. Given business. The structure is given in the question CH three. You can see double one ch two and three. We will meet them here. This one is sp hybridized and has a fast carbon atom.
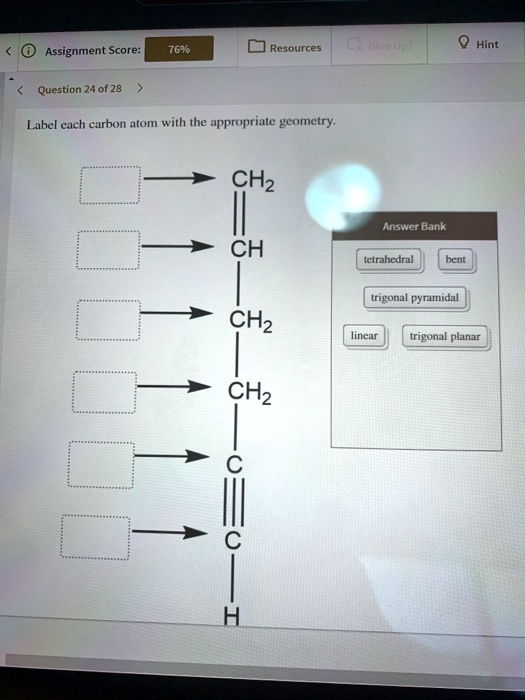
Label Each Carbon Atom With The Appropriate Geometry. The hybridizations of each carbon of the molecule are as follows If the carbon has 4 single bonds, then hybridization is. If the carbon has one double bond and two single bonds, then hybridization is. If the carbon has one triple bond or two double bonds, then hybridization is. Make sure the carbon has hybridization if it has two double bonds.
Label Each Carbon Atom With The Appropriate Hybridization. Label Each Carbon Atom With The Appropriate Hybridization. - 30086661. skittlepower3974 skittlepower3974 4 weeks ago Chemistry High School answered • expert verified Label Each Carbon Atom With The Appropriate Hybridization. 1 See answer Advertisement
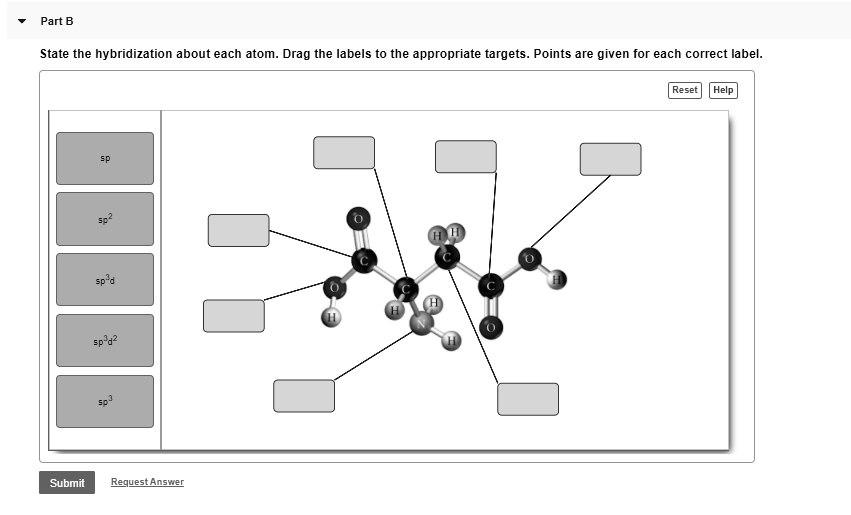
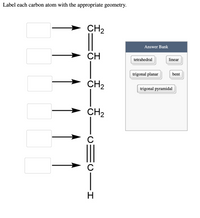
Question: Label each carbon atom with the appropriate geometry. Question: Label each carbon atom with the appropriate geometry. Label each carbon atom with the appropriate geometry. Show transcribed image text Expert Answer 98% (83 ratings) The hybridiz … View the full answer Transcribed image text: Label each carbon atom with the appropriate geometry. Previous question Next question
What is the hybridization of each atom in this molecule? More free chemistry help videos: is the easiest way to figure out how each atom's orbitals are hybrid...
Question: Label each carbon atom with the appropriate hybridization ... Label each carbon atom with the appropriate hybridization. sp,sp2 or sp3 Show transcribed image text Expert Answer 98% (94 ratings) Draw the structure of given molecule, mark the carbon atom as A, B, C, D, and E. Show the hybridization for the carbon marked A as shown below:The st … View the full answer Transcribed image text:
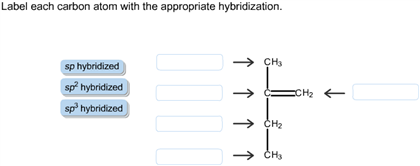
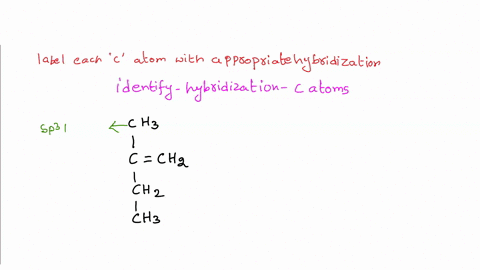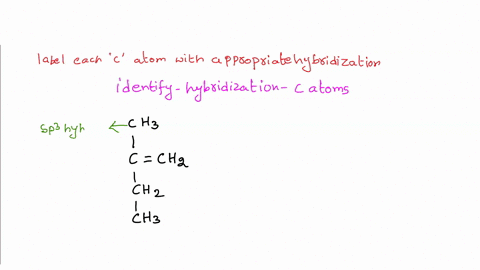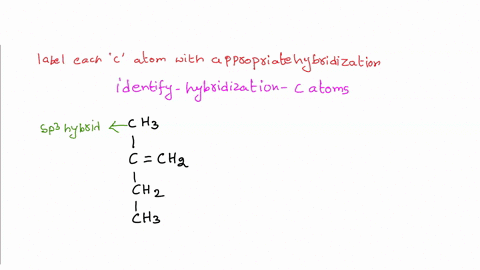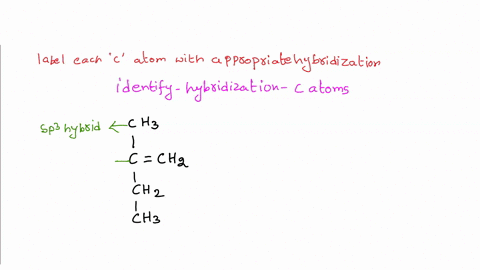
Solved Label each carbon atom with the appropriate | Chegg.com Label each carbon atom with the appropriate hybridization. sp hybridized > CH3 > -CH2 { spr hybridized sp3 hybridized * * * * > CH2 > CH3 This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer
Label each carbon atom with the appropriate geometry. - Course Hero Before overlapping with 1s orbital of hydrogen, first, the atomic orbitals of carbon undergoes hybridization to form hybrid orbitals. The hybrid orbitals of ...
What are hybridisation states of each carbon atom in the following ... The hybridisation states of each carbon atom in the following compounds are given below. ... sp2 Hybridization. 10 mins. sp3, sp3d and sp3d2 Hybridization. 22 mins. sp3d3 Hybridization. 11 mins. Shortcuts & Tips . Problem solving tips > Mindmap > Important Diagrams > Common Misconceptions >
Hybridization of Carbon - Molecular Geometry and Bond Angles - BYJUS Carbon can have an sp hybridization when it is bound to two other atoms with the help of two double bonds or one single and one triple bond. When the hybridization occurs the molecules have a linear arrangement of the atoms with a bond angle of 180°. Example: Hybridization of CO 2. 2. sp2 Hybridization
(Get Answer) - Label each carbon atom with the appropriate ... Label each carbon atom with the appropriate hybridization. sp,sp2 or sp3 Label each carbon atom with the appropriate hybridization. sp,sp2 or sp3 Nov 18 2022 08:12 AM
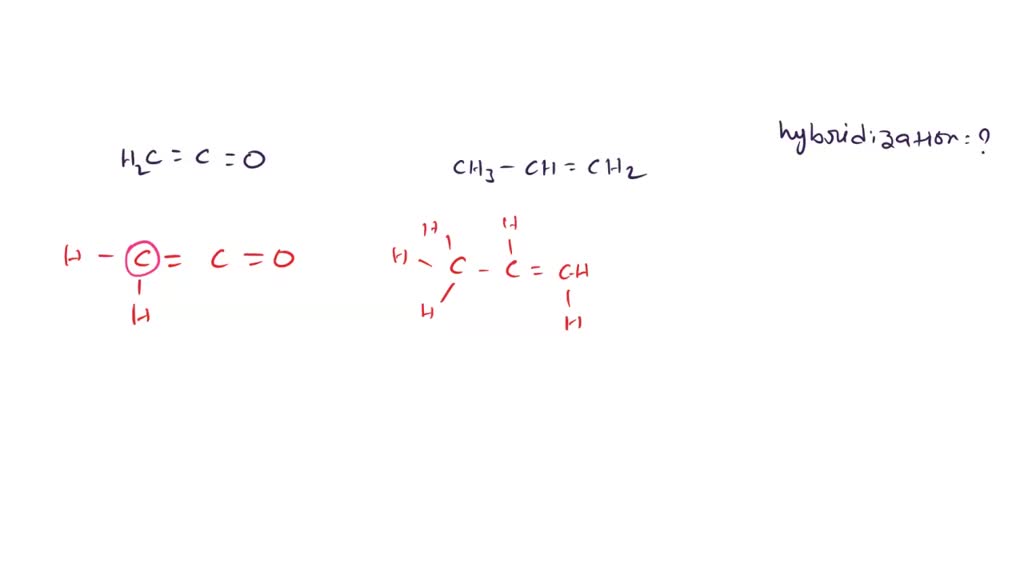
What are hybridisation states of each carbon atom in the following compounds ?a. CH2=C=O b. CH3CH=CH2















Post a Comment for "44 label each carbon atom with the appropriate hybridization"